Which of the Following Best Describes a Single Replacement Reaction
F H2 I2 2HI G 2NaCl 2Na Cl2 H NaF HCl HF NaCl. A compound breaks into separate elements.
Single Replacement Reactions Article Khan Academy
See answers 2 Best Answer.

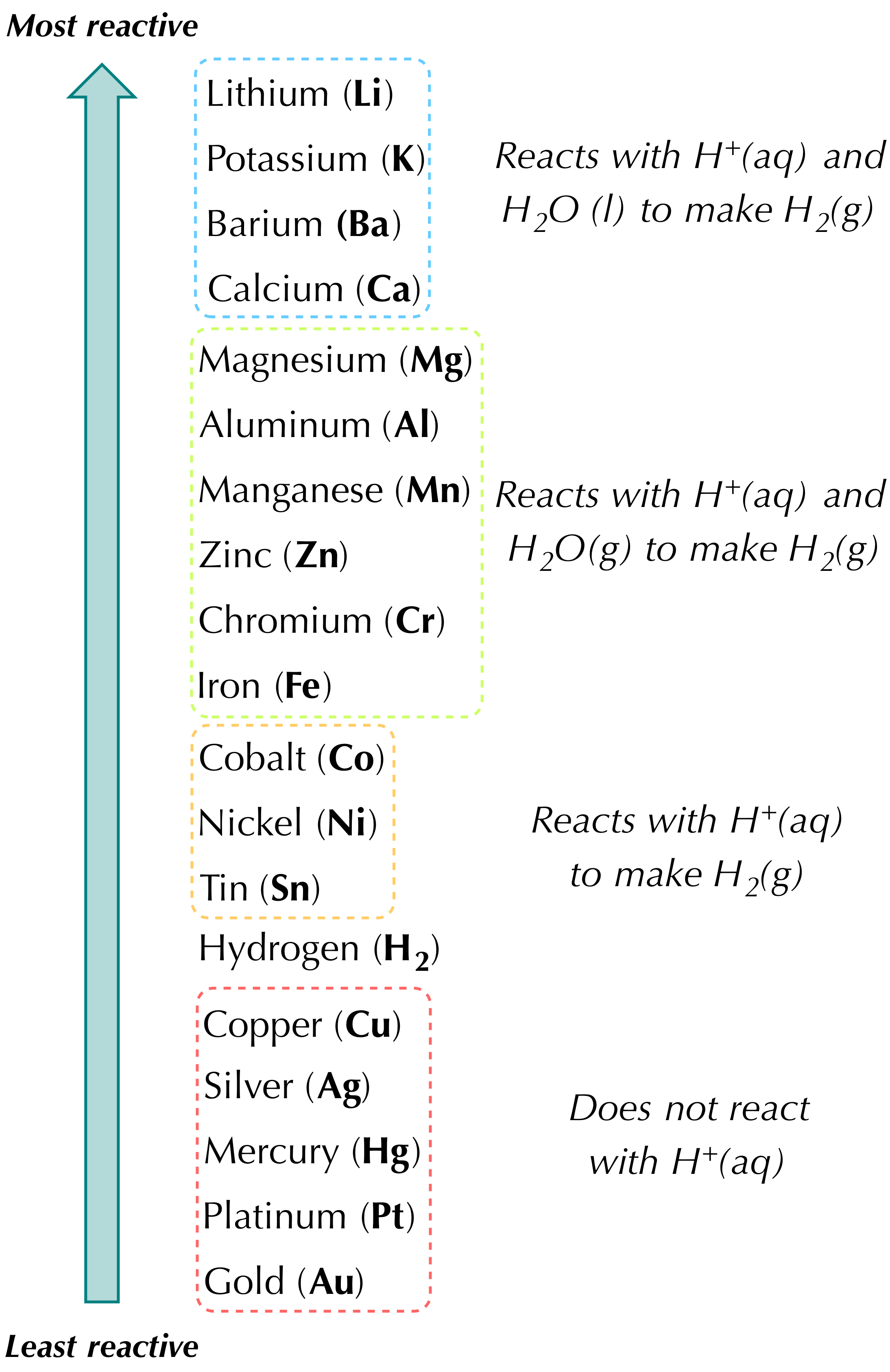
. A lone element takes the place of a different element in a compound. Note the arrow has this symbol Δ over it Δ Na2CO3s Na2Os CO2g Sodium carbonate is heated to give sodium oxide and carbon dioxide. The common non-metals in single replacement reactions are the group 17 elements which generally form anions with a 1- charge.
Which of the statements below best describes the following reaction. HClaq KOHaq KClaq H2Ol. What type of chemical reaction is illustrated in the following example.
Sodium carbonate decomposes with heat. A solution of silver nitrate is mixed with a solution of sodium chloride resulting in a precipitate of silver chloride and a solution of sodium nitrate. Which of the following best describes a single replacement reaction.
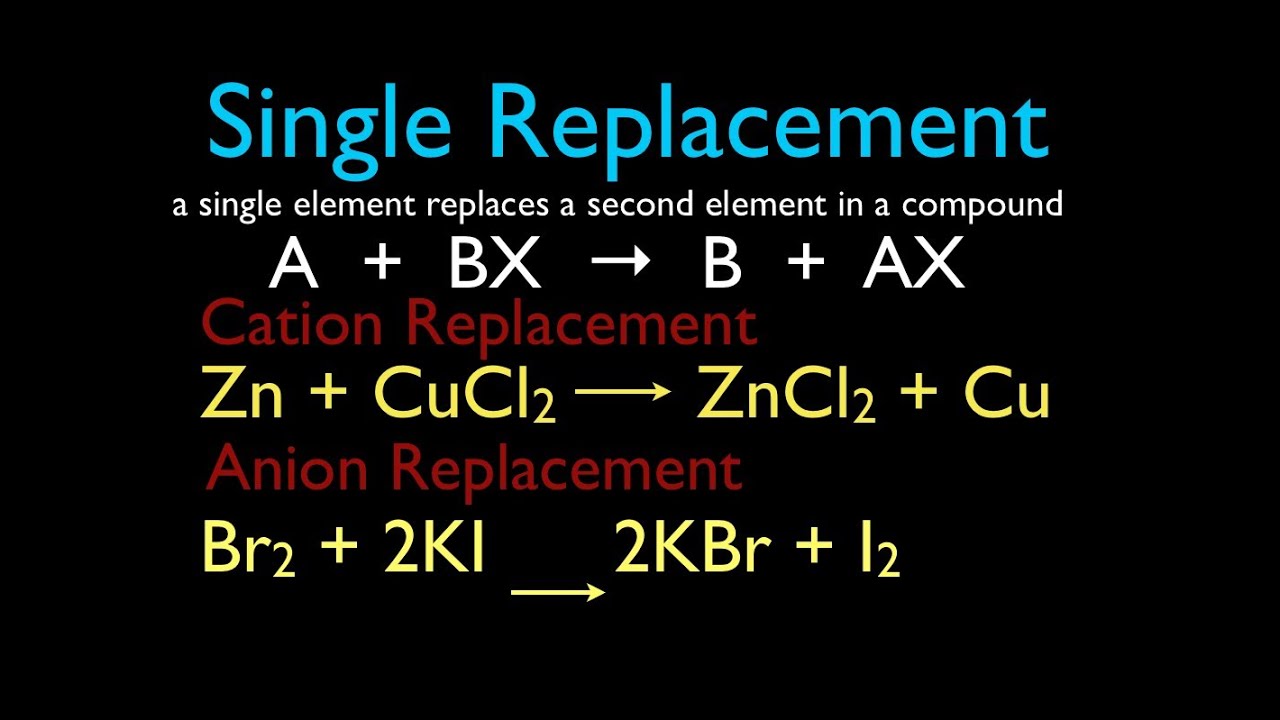
A single-replacement reaction also called a single-displacement reaction is one in which a pure element and a compound react chemically so that the products include another pure element and a different compound via exchanging of two species. What are the products from the following single-replacement reaction. Solid sodium carbonate is heated to give solid sodium oxide and carbon dioxide gas.
Two elements switch places in a compound. F H2SO4 CaOH2 CaSO4 H2O. Which of the following best represents the reaction between sulfuric acid and calcium hydroxide.
Which of the statements below best describes the following reaction. Zns CuSO4aq - Cu and ZnSO4. One element takes the place of another in a compound.
In other words it is where an element reacts with a compound and replaces one component of the compound. The following reaction is a redox reaction. Which of the statements below best describes the following reaction.
Which of the following best describes a double-replacement reaction. CaC 2 s H 2 Ol HCCHg CaOs Ans. Combination reaction C single-replacement reaction D double-replacement reaction E neutralization reaction.
The reaction of 50 mol of Fe CO5 80 mol of PF3 and 60 mol of H2 will release ________ mol of CO. Consider the following equation. C Single replacement D Combustion.
Predict the products of the following single replacement reaction. Balance polyatomic ions as a single unit check each reactant and product to verify the coefficients. One compound splits up forming two or more elements or new compounds.
Which reaction type best describes the. Two elements combine to form a compound. One element takes the place of another in a compound.
Atoms in one compound switch places with atoms in another compound. What will happen to the height h of the. Fes CuSO.
Which of these reactions shows simple chemical decomposition. Hydrogen usually forms the cation in a single replacement reaction. Two elements join together to form a new compound.
Which of the following describes a single-replacement reaction. Describe the procedure used to make 30 liters of a 20 M KCl solution starting with solid KCl and water. Mathrm Zn s2 mathrm HCl a q rightarrow mathrm ZnCl_ 2 a qmathrm H_ 2 g The reaction can be made to occur more slowly by 1 raising the temperature and using a single piece of zinc rather than powdered zinc of the same mass 2 lowering the.
The class of this reaction is.

A Synthesis Reaction Is A Type Of Reaction In Which Multiple Reactants Combine To Form A Single Product Synthesis React Chemical Reactions Chemistry Synthesis

Single Replacement Reaction Definition And Examples
Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube
0 Response to "Which of the Following Best Describes a Single Replacement Reaction"
Post a Comment